Photo taken in Monteverde, Costa Rica. Author: Cody Hinchliff, 2004. Licensed under CC BY-SA 3.0
Spend enough time around various Bromeliads and you will undoubtedly notice that some species have a rather gooey inflorescence. Indeed, floral mucilage is a well documented phenomenon within this family, with something like 30 species known to exhibit this trait. It is an odd thing to experience to say the least.
The goo takes on an interesting consistency. It reminds me a bit of finding frog spawn as a kid. Their brightly colored flowers erupt from this gooey coating upon maturity and the seeds of some species actually develop within the slimy coating. Needless to say, the presence of mucilage in these genera has generated some attention. Why do these plants do this?
Some have suggested that it is a type of reward for visiting pollinators. Analysis of the goo revealed that it is 99% water and 1% carbohydrate matrix with no detectable sugars or any other biologically useful compounds. As such, it probably doesn't do much in the way of attracting or rewarding flower visitors. Another hypothesis is that it could offer antimicrobial properties. Bromeliads are most often found in warm, humid climates where fungi and bacteria can really do a number. Again, no antimicrobial compounds were discovered nor did the mucilage show any sort of growth inhibition when placed in bacterial cultures.
It is far more likely that the mucilage offers protection from hungry herbivores. Flowers are everything to a flowering plant. They are, after all, the sexual organs. They take a lot of energy to produce and are often brightly colored, making them prime targets for a meal. Anything that protects the flowers during development would be a boon for any species. Indeed, it appears that the mucilage acts as a physical barrier, protecting the developing flowers and seeds. One study found that flowers protected by mucilage received significantly less damage from weevils than those without mucilage.
The mucilage could also provide another benefit to Bromeliads. Because these plants rely on water stored in the middle of their rosette (the tank, as it is sometimes called), some species may also gain a nutritional benefit as well. Bromeliad flowers emerge from this central tank so anything that gets stuck in the mucilage may eventually end up decomposing in the water. Since nutrients are absorbed along with the water, this could be an added meal for the plant. To date, this has not been confirmed. More work is needed before we can say for sure.
Photo Credit: [1]
Further Reading: [1] [2] [3] [4]









![A) Section of the fossil rhizome. B-J) Exquisitely preserve cellular details [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1496194937862-W7T6QUQJO62LM6E38UBP/image-asset.jpeg)




![Howea belmoreana and Howea forsteriana [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1493668069898-57NESX2V52YKJ81V4I12/nature04566-f1.2.jpg)





![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1490721649685-7VDQ5S929EILAFUA4HS1/image-asset.png)

![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1487096194923-6C9XB08WH90OLNUZFVD0/image-asset.jpeg)

![[SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1486578795815-PMLOXE5169FQ31BLAB9V/image-asset.jpeg)

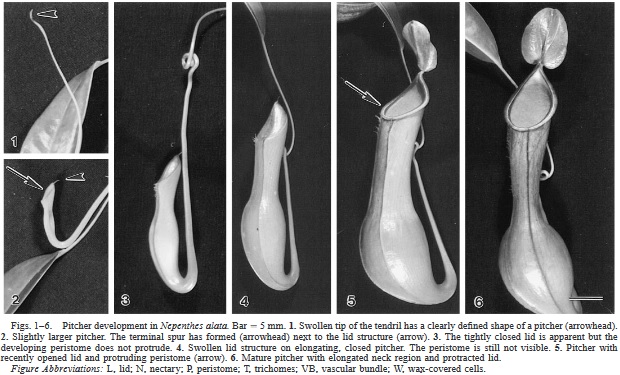
![Photo by Monro & Wei [SOURCE]](https://images.squarespace-cdn.com/content/v1/544591e6e4b0135285aeb5b6/1482084165365-K9E5AXN7TT1EG4WLP8BR/image-asset.jpeg)